Initiating and titrating ENTRESTO® to target dose1
ENTRESTO® should only be initiated in clinically stable patients whose baseline systolic blood pressure, serum potassium and renal function are at acceptable levels.
Patients with:
-
•
Prior ACE inhibitor or ARB at less than guideline-recommended doses
-
•
Risk for hypotension (≥75 years old, low SBP)
-
•
Moderate hepatic impairment (Child-Pugh B)

Patients with:
-
•
Prior ACE inhibitor or ARB at guideline-recommended doses
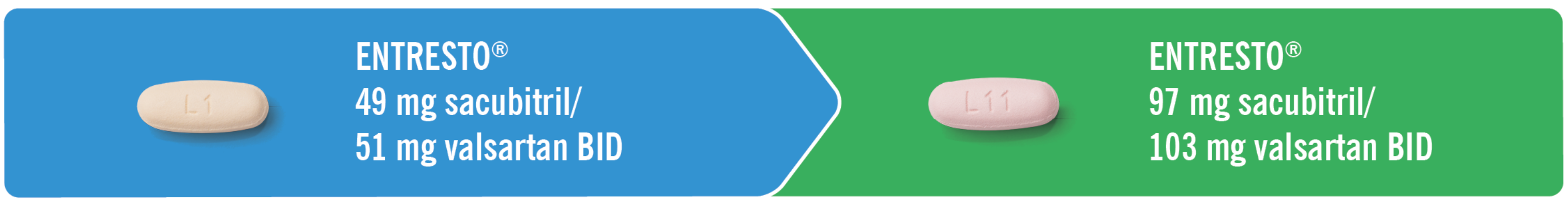
Stop ACE inhibitor therapy for a 36-hour washout.1
ENTRESTO® must not be started until 36 hours have passed following discontinuation of ACE inhibitor therapy.1
If patients experience tolerability issues, e.g., symptomatic hypotension or hyperkalemia, consideration should be given to temporary down-titration or treatment interruption of ENTRESTO®.1
ENTRESTO® should normally be used in conjunction with other medical treatment for HF, including diuretics, beta-blockers, and mineralocorticoid receptor antagonists, as appropriate and as tolerated.1
ENTRESTO® must not be administered with any drug formulation containing an ACE inhibitor due to the risk of angioedema and should not be co-administered with any other drug formulation containing an ARB.1
ENTRESTO® should be used in place of an ACE inhibitor or ARB.1
ACE: angiotensin-converting-enzyme; ARB: angiotensin receptor blocker.
